WalkAide – Option for Correcting Foot Drop
 The WalkAide is an advanced therapeutic concept combining the bracing role of orthotics with the muscle restoration of functional electrical stimulation (FES) for patients suffering from foot drop, the inability to properly lift (dorsiflex) the forefoot during ambulation. Foot drop is a common outcome of multiple sclerosis, cerebral palsy, stroke, traumatic brain injury, and spinal cord injury.
The WalkAide is an advanced therapeutic concept combining the bracing role of orthotics with the muscle restoration of functional electrical stimulation (FES) for patients suffering from foot drop, the inability to properly lift (dorsiflex) the forefoot during ambulation. Foot drop is a common outcome of multiple sclerosis, cerebral palsy, stroke, traumatic brain injury, and spinal cord injury.
Common manifestations are toe-dragging in swing phase and foot slap at the beginning of stance phase. Patients with foot drop often compensate with exaggerated high-stepping ambulation known as steppage gait.
 The WalkAide surmounts dorsiflexor weakness or paralysis by stimulating the peroneal nerve at the appropriate point in the gait cycle to lift the forefoot, assuring ground clearance and providing for a normal heel-to-toe rollover. The result is a more natural, smoother, safer, and more energy-efficient gait. In recreating the natural nerve-to-muscle response, the WalkAide not only corrects for biomechanical dysfunction but may improve circulation, reduce atrophy and increase joint range of motion.
The WalkAide surmounts dorsiflexor weakness or paralysis by stimulating the peroneal nerve at the appropriate point in the gait cycle to lift the forefoot, assuring ground clearance and providing for a normal heel-to-toe rollover. The result is a more natural, smoother, safer, and more energy-efficient gait. In recreating the natural nerve-to-muscle response, the WalkAide not only corrects for biomechanical dysfunction but may improve circulation, reduce atrophy and increase joint range of motion.
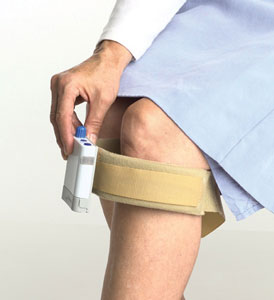
The FDA-approved Walk-Aide consists of a battery-operated electrical stimulator, two electrodes and electrode leads packaged into a small case, which is held in position by a cuff on the affected leg just below the knee. As compared to an ankle-foot orthosis (AFO), the WalkAide provides some patients with better gait, increased comfort and a more natural appearance.
Walk-Aid contraindications include lower motor neuron and/or peripheral nerve damage; secondary complications of knee, back or hip surgery; leg trauma; sciatica; peripheral neuropathy; spinal stenosis; post-polio syndrome and Guillain-Barre. The WalkAide should not be used by individuals wearing a pacemaker or those subject to seizures.

